LOS ANGELES, Feb. 14, 2024 /PRNewswire/ — QT Medical, a medtech company focusing on improving cardiac care with electrocardiogram (ECG or EKG), announces that it has received 510(k) clearance from the FDA for PCA 500™ use in acute care. PCA 500 was first 510(k) cleared in 2018 for professional and personal use by adults in non-acute care settings, including patient’s homes. In 2022, QT Medical received 510(k) clearance of PCA 500 use in infants, children and adolescents. The new FDA clearance expands PCA 500’s indication for use to all healthcare settings, including: hospitals, intensive care units, emergency rooms and ambulances.
The development of PCA 500 was initially funded by the National Institutes of Health (NIH) for an easy-to-use ECG. With its patented electrodes (QHeart™ sensor) and compact recorder, PCA 500 offers digital, mobile and cloud-based ECG management solution. PCA 500 is widely used by airlines, telehealth practice, clinics, skilled nursing facilities, and in clinical trials and schools.
“We are extremely excited about the potential of PCA 500 in modernizing ECG technology and improving heart health for millions of patients. With the FDA clearance for use in acute care, such as ambulances and emergency rooms, PCA 500 brings significant values to where efficiency and accuracy are needed the most.” said Dr. Ruey-Kang Chang, CEO of QT Medical, Inc.
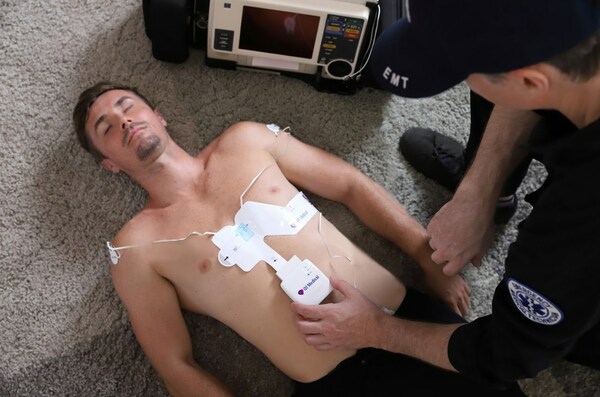
QT Medical plans to promote the use of PCA 500 in prehospital care by paramedics and in emergency rooms. Prehospital ECG by paramedics is known to decrease the “door-to-balloon” time thereby increasing the chance for better patient outcomes. In a busy emergency room, it may be challenging to comply with the ACC/AHA guidelines of keeping the “door-to-ECG” time (arrival of a patient with chest pain to when an ECG is recorded) under 10 min. Thanks to the pre-positioned QHeart sensors, placing ECG sensor is quick, simple, and eliminating the possibility of leads reversal. Automated periodic recording coupled with serial comparison of ECG tracings make it possible to detect subtle clinical changes for early diagnosis and treatment.
QT Medical’s PCA 500 and QHeart sensor enable quick and accurate ECG acquisition, and immediate transmission to a secure cloud server, which can greatly reduce the ‘door-to-balloon’ time.
QT Medical’s PCA 500 and QHeart sensor enable quick and accurate ECG acquisition, and immediate transmission to a secure cloud server, which can greatly reduce the ‘door-to-balloon’ time.
About QT Medical
QT Medical is a medtech company with a focus on high quality 12-lead diagnostic electrocardiogram (ECG) for use by healthcare professionals and patients. Cleared by the FDA and CE marked, PCA 500 is the world’s most compact and versatile 12-lead ECG system. With its simplicity, ease of use, mobile technology and cloud management, PCA 500 brings hospital-grade ECG to all healthcare facilities and millions of homes for better cardiac care. For more information, please visit: www.qtmedical.com
View original content to download multimedia: Read More